Introduction:Antiphospholipid syndrome (APS) is characterized by arterial and/or venous thrombosis in the presence of antiphospholipid antibodies. Recently, derangement in the complement pathway has been implicated in APS pathophysiology. Refractory cases of APS with recurrent and potentially fatal thrombosis have prompted use of the monoclonal antibody eculizumab, which inhibits generation of the terminal complement complex. We present the successful use of eculizumab in controlling and preventing recurrent thrombosis in a refractory case of APS.
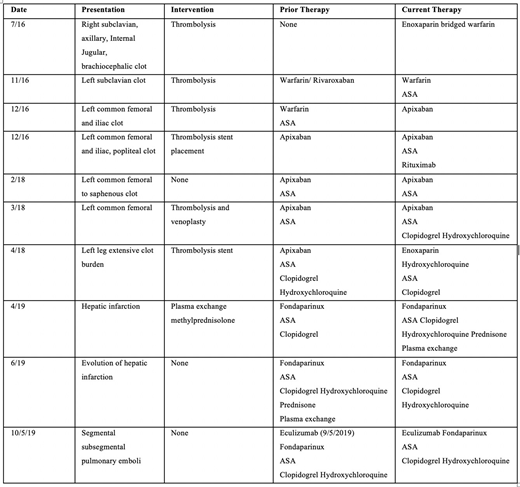
Case Description:An 18-year-old female received a diagnosis of antiphospholipid syndrome after developing extensive unprovoked deep vein thrombosis of axillary, inferior vena caval and brachiocephalic veins. Thrombophilia evaluation revealed triple positivity for lupus anti-coagulant (LA), beta-2 glycoprotein I (GP) IgG 84.9 SGU Units, IgM 76.5 SMU Units, IgA 66.7 SAU Units and strongly positive anti-cardiolipin (aCL) antibodies (each >>40U/mL) with persistent positive titers after 12 weeks of initial evaluation. She was refractory with trials of multiple anticoagulants alone and with antiplatelet and adjunctive therapies. Anticoagulants used were enoxaparin, fondaparinux, apixaban, rivaroxaban, and warfarin; antiplatelet agents used were aspirin and clopidogrel; and adjunctive therapies included hydroxychloroquine, immunosuppression with steroids and rituximab, and plasmapheresis. Despite these interventions, she continued to develop recurrent thrombosis of subclavian, femoral, common femoral, iliac, popliteal and saphenous veins. She additionally developed a potentially life-threatening hepatic infarction and pulmonary embolism, and 6 weeks of plasma exchange failed to decrease antiphospholipid antibody titers. Following this event, eculizumab (600mg weekly x 4 weeks, followed by 900mg once on week 5, followed by 900mg every 2 weeks) was initiated in combination with fondaparinux, aspirin, clopidogrel, and hydroxychloroquine. She has remained on this regimen with additional anticoagulation and antiplatelet therapy without recurrence of thrombosis over the subsequent year.
Discussion:Recent evidence has shown complement activation playing a role in pathophysiology of antiphospholipid syndrome. This is thought to occur through C3 and C5a causing platelet and endothelial activation, leading to microvascular thrombosis. Histological evidence of such processes is supported by the demonstration of anti-beta-2 GP1 IgG-C5b-9 immune complexes in microvascular organ thrombi in individuals with APS leading to thrombotic microangiopathy and multiorgan failure. Such evidence of complement induced thrombosis in antiphospholipid syndrome led us to use eculizumab following failure of available therapies including anticoagulation, antiplatelet therapy, immune modulation and plasmapheresis in a potentially life-threatening event. Absence of recurrent thrombotic events over approximately one year with eculizumab combined with anticoagulation and antiplatelet therapy highlights the potential role of complement inhibition in preventing thrombosis in APS. While the role of complement in thrombosis continues to be elucidated, we have observed no decline in antiphospholipid antibody levels in a span of one year in our patient. Importantly, there has been no recurrent thrombosis during this time. Our case suggests that eculizumab may have a role as a therapeutic option in refractory thrombosis in APS.
Chronology of Events:Eculizumab initiated on 9/5/2019. No evidence of venous or arterial thrombosis since 10/2019 to present.
Tarantino:Pfizer:Other;Genentech:Membership on an entity's Board of Directors or advisory committees;Octapharma:Membership on an entity's Board of Directors or advisory committees;Dova:Membership on an entity's Board of Directors or advisory committees;CDC:Membership on an entity's Board of Directors or advisory committees;Grifols:Membership on an entity's Board of Directors or advisory committees, Speakers Bureau;Amgen:Membership on an entity's Board of Directors or advisory committees;Biomarin:Membership on an entity's Board of Directors or advisory committees;NovoNordisk:Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau;Takeda:Research Funding;HRSA:Membership on an entity's Board of Directors or advisory committees;Spark:Membership on an entity's Board of Directors or advisory committees;Sobi:Membership on an entity's Board of Directors or advisory committees.McCrae:Rigel:Consultancy;Novartis:Honoraria;Momenta Pharmaceuticals:Consultancy;Dova:Consultancy.Chaturvedi:Sanofi:Honoraria, Membership on an entity's Board of Directors or advisory committees;Alexion:Honoraria, Membership on an entity's Board of Directors or advisory committees.Roberts:Octapharma:Consultancy, Speakers Bureau;uniQure:Consultancy;Sanofi:Consultancy, Speakers Bureau;Novo Nordisk:Consultancy, Speakers Bureau;Pfizer:Consultancy;Takeda:Consultancy, Research Funding, Speakers Bureau.
eculizumab for treatment of refractory thrombosis in antiphospholipid syndrome
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal